Showing posts with label Chemistry. Show all posts
Showing posts with label Chemistry. Show all posts
Sunday, January 23, 2011
Sunday, January 9, 2011
Selected Quia Games and Quizzes
http://www.quia.com/shared/
SPANISH:
Spanish pronouns: http://www.quia.com/mc/322547.html
Adjectives:
http://www.quia.com/mc/66063.html
Food and Drinks: http://www.quia.com/cb/28652.html
Body Parts:
http://www.quia.com/quiz/794975.html?AP_rand=578159630
Colors:
http://www.quia.com/hm/163554.html
Days of the Week: http://www.quia.com/jw/116339.html?AP_rand=1357339885
SCIENCE:
Chemistry - common elements:
http://www.quia.com/mc/65904.html
Atomic Structure: http://www.quia.com/rr/70834.html
30 Elements' Symbols (Matching Game)
http://www.quia.com/jg/907334.html
40 Elements' Symbols (Matching Game)
http://www.quia.com/jg/611032.html
Properties of Matter
http://www.quia.com/jg/611032.html
Periodic Table of Elements (Scavenger HUnt) check websites!
http://www.quia.com/sh/9629.html?AP_rand=642082197
Elemental Table (Rags to Riches)
http://www.quia.com/rr/41449.html
Atoms and Elements: http://www.quia.com/rr/41449.html
CIVICS:
Bill of Rights (First 10 Amendments)
http://www.quia.com/pp/43250.html?AP_rand=1395570334
Rags to Riches version: http://www.quia.com/rr/686920.html
Matching Version: http://www.quia.com/mc/2049564.html
Bill of Rights Vocabulary: http://www.quia.com/rr/686921.html
5th Grade - Branches of Government:
http://www.quia.com/rr/103659.html
Election Process: http://www.quia.com/rr/580312.html
How a Bill Becomes a Law:
http://www.quia.com/rd/194382.html?AP_rand=300886918
Constitution and History:
http://www.quia.com/jg/2068207.html
MATH:
5th grade math vocab
http://www.quia.com/jg/2068207.html
WORLD HISTORY:
Short ancient timeline
http://www.quia.com/rd/37561.html?AP_rand=875454615
SPANISH:
Spanish pronouns: http://www.quia.com/mc/322547.html
Adjectives:
http://www.quia.com/mc/66063.html
Food and Drinks: http://www.quia.com/cb/28652.html
Body Parts:
http://www.quia.com/quiz/794975.html?AP_rand=578159630
Colors:
http://www.quia.com/hm/163554.html
Days of the Week: http://www.quia.com/jw/116339.html?AP_rand=1357339885
SCIENCE:
Chemistry - common elements:
http://www.quia.com/mc/65904.html
Atomic Structure: http://www.quia.com/rr/70834.html
30 Elements' Symbols (Matching Game)
http://www.quia.com/jg/907334.html
40 Elements' Symbols (Matching Game)
http://www.quia.com/jg/611032.html
Properties of Matter
http://www.quia.com/jg/611032.html
Periodic Table of Elements (Scavenger HUnt) check websites!
http://www.quia.com/sh/9629.html?AP_rand=642082197
Elemental Table (Rags to Riches)
http://www.quia.com/rr/41449.html
Atoms and Elements: http://www.quia.com/rr/41449.html
CIVICS:
Bill of Rights (First 10 Amendments)
http://www.quia.com/pp/43250.html?AP_rand=1395570334
Rags to Riches version: http://www.quia.com/rr/686920.html
Matching Version: http://www.quia.com/mc/2049564.html
Bill of Rights Vocabulary: http://www.quia.com/rr/686921.html
5th Grade - Branches of Government:
http://www.quia.com/rr/103659.html
Election Process: http://www.quia.com/rr/580312.html
How a Bill Becomes a Law:
http://www.quia.com/rd/194382.html?AP_rand=300886918
Constitution and History:
http://www.quia.com/jg/2068207.html
MATH:
5th grade math vocab
http://www.quia.com/jg/2068207.html
WORLD HISTORY:
Short ancient timeline
http://www.quia.com/rd/37561.html?AP_rand=875454615
Monday, September 27, 2010
Periodic Table Fun
A cool photographic Periodic Table: http://periodictable.com/
A fun Quiz
Rows of elements are called periods. The period number of an element signifies the highest unexcited energy level for an electron in that element. The number of elements in a period increases as you move down the periodic table because there are more sublevels per level as the energy level of the atom increases.
Columns of elements help define element groups. Elements within a group share several common properties. http://chemistry.about.com/library/blperiodictablekids.htm
A more complex way to explain it:
A fun Quiz
Periodic Table of Elements The periodic table is the most important reference a chemist has because it puts all the known elements into a meaningful pattern. Elements are arranged left to right and top to bottom in order of increasing atomic number. This order generally goes with increasing atomic mass. Click on an element for more information (clicks take you to the Los Alamos National Laboratory site):
The different rows of elements are called periods. The period number of an element signifies the highest energy level an electron in that element occupies (in the unexcited state). The number of elements in a period increases as one moves down the periodic table because as the energy level of the atom increases, the number of energy sub-levels per energy level increases. In 1869, the Russian chemist Mendeleev noted that the repeating patterns of behavior could be arranged in a sequence of elements. This led to the first "Periodic Table" of the elements. Scientists and students who are familiar with the periodic table use the position in the table to extract information about individual elements. Chemistry in a Nutshell For a list of the element names and symbols in alphabetical order. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Another way to explain it:
The periodic table arranges the chemical elements into a pattern so that you can predict the properties of elements based on where they are located on the table. Elements are arranged from left to right and from top to bottom in order of increasing atomic number or number of protons in the element.Rows of elements are called periods. The period number of an element signifies the highest unexcited energy level for an electron in that element. The number of elements in a period increases as you move down the periodic table because there are more sublevels per level as the energy level of the atom increases.
Columns of elements help define element groups. Elements within a group share several common properties. http://chemistry.about.com/library/blperiodictablekids.htm
A more complex way to explain it:
The periodic table is a chart of the elements arranged according to
the periodic law discovered by Dmitri I. Mendeleev and revised by Henry G. J.
Moseley. In the periodic table the elements are arranged in columns and rows according to increasing atomic number (see the table entitled Periodic Table). The vertical columns, or groups, are numbered from I to VIII, with a final column numbered 0. Each group is divided into two categories, or families: one called the a series (the representative, or main group, elements); the other the b series (the transition, or subgroup, elements).
All the elements in a group have the same number of valence electrons and hence similar chemical properties.
The horizontal rows of the table are called periods. The elements of a period are characterized by the fact that they have the same number of electron shells; the number of electrons in these shells, which equals the element's atomic number, increases from left to right within each period.
In each period the lighter metals appear on the left, the heavier metals in the center, and the nonmetals on the right. Elements on the borderline between metals and nonmetals are called metalloids.Group Ia (with one valence electron) and group IIa (with two valence electrons) are called the alkali metals and the alkaline-earth metals, respectively.
Two series of elements branch off from group IIIb, which contains the transition elements, or transition metals; elements 57 to 71 are called the lanthanide series, or rare earths, and elements 89 to 103 are called the actinide series, or radioactive rare earths; a third group, the superactinide group (elements 122—153), is predicted to fall outside the main body of the table, but none of these has yet been synthesized or isolated.
The nonmetals in group VIIa (with seven valence electrons) are called the halogens.
The elements grouped in the final column have no valence electrons and are called the inert gases, or noble gases, because they react chemically only with extreme difficulty.
In a relatively simple type of periodic table, each position gives the name and chemical symbol for the element assigned to that position; its atomic number; its atomic weight (the weighted average of the masses of its stable isotopes, based on a scale in which carbon-12 has a mass of 12); and its electron configuration, i.e., the distribution of its electrons by shells.
The only exceptions are the positions of elements 103 through 116; complete information on these elements has not been compiled.
Larger and more complicated periodic tables may also include the following information for each element: atomic diameter or radius; common valence numbers or oxidation states; melting point; boiling point; density; specific heat; Young's modulus; the quantum states of its valence electrons; type of crystal form; stable and radioactive isotopes; and type of magnetism exhibited by the element (paramagnetism or diamagnetism).
See P. W. Atkins, The Periodic Kingdom: A Journey into the Land of Chemical Elements (1997).
Wednesday, February 10, 2010
Meet the Elements
Iron is a metal
You see it every day
Oxygen eventually
Will make it rust away
Carbon in it's ordinary form is coal
Crush it together and diamonds are born
Come on come on and meet the elements
May I introduce you to our friends the elements?
Like a box of paints
that are mixed to make every shade
They either combine to make a chemical compound
Or stand alone as they are
Neon's the gas that lights up the sign for a pizza place
The coins that you pay with are copper, nickel and zinc
Silicon and oxygen make concrete, bricks and glass
Now add some gold and silver for some pizza place class
Come on come on and meet the elements
I think you should check out the ones they call the elements
Like a box of paints that are mixed to make every shade
They either combine to make a chemical compound
Or stand alone as they are
Team up with other elements
Making compounds when they combine
Or make up a simple element
Formed out of atoms of the one kind.
Balloons are full of helium
And so is every star
Stars are mostly hydrogen
Which may someday fuel your car
Hey who let in all these elephants?
Did you know that elephants are made of elements?
Elephants are mostly made of four elements
And every living thing is mostly made of four elements
Plants, bugs, birds, fish, bacteria and men
Are mostly carbon, hydrogen, nitrogen and oxygen
Come on come on and meet the elements
You and I are complicated but we're made of elements
Like a box of paints that are mixed to make every shade
They either combine to make a chemical compound
Or stand alone as they are
Team up with other elements
Making compounds when they combine
Or make up a simple element
Formed out of atoms of the one kind.
Come on come on and meet the elements
Check out the ones they call the elements
Like a box of paints that are mixed to make every shade
They either combine to make a chemical compound
Or stand alone as they are
SOURCES: http://lyrics.url.com/show/6645/they-might-be-giants/meet-the-elements-lyrics
and
http://www.sciencefriday.com/program/archives/200911273
and
http://www.youtube.com/watch?v=d0zION8xjbM&feature=player_embedded
Friday, January 15, 2010
Periodic Table Memory Pegs
![1 Hydrogen (H): Hydrant [blue], 1-shaped 1 Hydrogen (H): Hydrant [blue], 1-shaped](http://www.johnpratt.com/atomic/images/01hydrogen.gif) | ![2 Helium (He): Helium Balloon [silver], 2nd place 2 Helium (He): Helium Balloon [silver], 2nd place](http://www.johnpratt.com/atomic/images/02helium.gif) | ||||||||||||||||
![3 Lithium (Li): Lithium Battery [lilac], 3-volt 3 Lithium (Li): Lithium Battery [lilac], 3-volt](http://www.johnpratt.com/atomic/images/03lithium.gif) | ![4 Beryllium (Be): Belt Buckle [pink], 4 beryls 4 Beryllium (Be): Belt Buckle [pink], 4 beryls](http://www.johnpratt.com/atomic/images/04beryllium.gif) | ![5 Boron (B): Box of Borax [brown], 5 lbs. 5 Boron (B): Box of Borax [brown], 5 lbs.](http://www.johnpratt.com/atomic/images/05boron.gif) | ![6 Carbon (C): Charcoal Pencil [black], 6-sided 6 Carbon (C): Charcoal Pencil [black], 6-sided](http://www.johnpratt.com/atomic/images/06carbon.gif) | ![7 Nitrogen (N): Dynamite [red], 7-shaped 7 Nitrogen (N): Dynamite [red], 7-shaped](http://www.johnpratt.com/atomic/images/07nitrogen.gif) | ![8 Oxygen (O): Life Preservers [white], 8-shaped 8 Oxygen (O): Life Preservers [white], 8-shaped](http://www.johnpratt.com/atomic/images/08oxygen.gif) | ![9 Fluorine (F): Fluoride Toothpaste [purple], 9-shaped 9 Fluorine (F): Fluoride Toothpaste [purple], 9-shaped](http://www.johnpratt.com/atomic/images/09fluorine.gif) | ![10 Neon (Ne): Neon Sign [orange], 10-shaped 10 Neon (Ne): Neon Sign [orange], 10-shaped](http://www.johnpratt.com/atomic/images/10neon.gif) | ||||||||||
![11 Sodium (Na): Narrow Soda Crackers [yellow], 11-shaped 11 Sodium (Na): Narrow Soda Crackers [yellow], 11-shaped](http://www.johnpratt.com/atomic/images/11sodium.gif) | ![12 Magnesium (Mg): Mug [green], 12 oz. 12 Magnesium (Mg): Mug [green], 12 oz.](http://www.johnpratt.com/atomic/images/12magnesium.gif) | ![13 Aluminum (Al): Aluminum Ladder [turquoise], unlucky 13 13 Aluminum (Al): Aluminum Ladder [turquoise], unlucky 13](http://www.johnpratt.com/atomic/images/13aluminum.gif) | | ![15 Phosphorus (P): Phosphorus Matches [red/white striped], book of 15 15 Phosphorus (P): Phosphorus Matches [red/white striped], book of 15](http://www.johnpratt.com/atomic/images/15phosphorus.gif) | ![16 Sulfur (S): Sulfuric Acid [pale yellow], 16 oz. 16 Sulfur (S): Sulfuric Acid [pale yellow], 16 oz.](http://www.johnpratt.com/atomic/images/16sulfur.gif) | ![17 Chlorine (Cl): Clorox [yellow-green], cleans 17 ways 17 Chlorine (Cl): Clorox [yellow-green], cleans 17 ways](http://www.johnpratt.com/atomic/images/17chlorine.gif) | ![18 Argon (Ar): Argon-filled Fluorescent Lights [sky blue], 18 watt 18 Argon (Ar): Argon-filled Fluorescent Lights [sky blue], 18 watt](http://www.johnpratt.com/atomic/images/18argon.gif) | ||||||||||
![19 Potassium (K): Kettle or Pot of ashes [gray], 19th Century 19 Potassium (K): Kettle or Pot of ashes [gray], 19th Century](http://www.johnpratt.com/atomic/images/19potassium.gif) | ![20 Calcium (Ca): Calcite Pearls [ivory], string of 20 20 Calcium (Ca): Calcite Pearls [ivory], string of 20](http://www.johnpratt.com/atomic/images/20calcium.gif) |  |  |  |  | 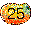 | 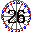 |  | ![28 Nickel (Ni): Nickel, richer in '28 [see 29] 28 Nickel (Ni): Nickel, richer in '28 [see 29]](http://www.johnpratt.com/atomic/images/28nickel.gif) |  |  |  |  |  |  | 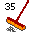 |  |
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
 |  |  |  | 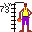 |  |  |  |  |  |  |  |  |  |  |  |  |  |
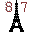 |  |  |  |  | |||||||||||||
 | 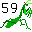 |  |  |  | 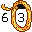 |  |  |  |  |  |  |  | 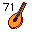 | ||||
 | 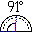 |  |  |  |  |  |  |  |  |  |  |  |  | ||||
©John P. Pratt, all rights reserved
Icons drawn by David R. Pratt
16 September 1997
updated 2 Feb 2006 to show data better with mouse
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Atomic Number Memory Pegs: 1-20
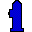 1. Hydrant (Hydrogen H). Blue for "first place" (blue ribbon) and for water, its principal compound; tall and thin, shaped like a "1". Hydrant sounds like hydrogen (both refer to water).
1. Hydrant (Hydrogen H). Blue for "first place" (blue ribbon) and for water, its principal compound; tall and thin, shaped like a "1". Hydrant sounds like hydrogen (both refer to water). 2. Helium Balloon (Helium He). Silver colored for "second place" (silver medal); a noble color for a noble gas.
2. Helium Balloon (Helium He). Silver colored for "second place" (silver medal); a noble color for a noble gas. 3. Lithium watch battery (Lithium Li), 3-volt. Lilac colored (sounds like lithium and all three natural minerals are lilac colored).
3. Lithium watch battery (Lithium Li), 3-volt. Lilac colored (sounds like lithium and all three natural minerals are lilac colored). 4. Beryl-studded Belt Buckle (Beryllium Be). Rectangular (4 sides), with four pink beryls (beryl is a pink gem: beryllium aluminum silicate).
4. Beryl-studded Belt Buckle (Beryllium Be). Rectangular (4 sides), with four pink beryls (beryl is a pink gem: beryllium aluminum silicate). 5. Box of Borax (Boron B). 5-pound box, dark brown, the color of pure boron (borax is hydrated sodium borate).
5. Box of Borax (Boron B). 5-pound box, dark brown, the color of pure boron (borax is hydrated sodium borate). 6. Charcoal pencil (Carbon C). Black, the color of charcoal and also of graphite (carbon) in pencil leads. Hexagonal (6-sided).
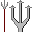
6. Charcoal pencil (Carbon C). Black, the color of charcoal and also of graphite (carbon) in pencil leads. Hexagonal (6-sided). 7. Dynamite, or Nitroglycerin (Nitrogen N). Two red sticks, shaped like a "7" (most explosives contain nitrogen).
7. Dynamite, or Nitroglycerin (Nitrogen N). Two red sticks, shaped like a "7" (most explosives contain nitrogen). 8. Oxygen-filled Lifepreservers (Oxygen O). White for air, which preserves life. Two of them touching, shaped like an "8".
8. Oxygen-filled Lifepreservers (Oxygen O). White for air, which preserves life. Two of them touching, shaped like an "8". 9. Fluoride Toothpaste (Fluorine F). Purple, the color of the mineral fluorite (calcium fluoride), and shaped like a 9.
9. Fluoride Toothpaste (Fluorine F). Purple, the color of the mineral fluorite (calcium fluoride), and shaped like a 9.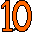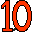 10. Neon Sign (Neon Ne). Shaped like a "10," flashing orange, like the orange/red color of neon in neon signs.
10. Neon Sign (Neon Ne). Shaped like a "10," flashing orange, like the orange/red color of neon in neon signs. 11. Narrow Soda Crackers (Sodium Na). Two of them, bright yellow, shaped like an "11," like the bright yellow sodium D spectral lines. Sodium is named for soda (sodium carbonate).
11. Narrow Soda Crackers (Sodium Na). Two of them, bright yellow, shaped like an "11," like the bright yellow sodium D spectral lines. Sodium is named for soda (sodium carbonate). 12. Mug. (Magnesium Mg) 12 oz. green mug made of olivine (magnesium silicate), filled with milk of magnesia (magnesium hydroxide). Magnesium in chlorophyll gives plants their green color.
12. Mug. (Magnesium Mg) 12 oz. green mug made of olivine (magnesium silicate), filled with milk of magnesia (magnesium hydroxide). Magnesium in chlorophyll gives plants their green color. 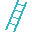 13. Aluminum Ladder (Aluminum Al). Unlucky to walk under, and 13 is unlucky. Turquoise (blue-green) colored (turquoise is hydrated aluminum phosphate.
13. Aluminum Ladder (Aluminum Al). Unlucky to walk under, and 13 is unlucky. Turquoise (blue-green) colored (turquoise is hydrated aluminum phosphate. 15. Phosphorus Matches (Phosphorus P). Red/white striped book of 15, heads white, striker red (there are both white and red forms of phosphorus). The matches could light the dynamite (nitrogen) in the periodic table.
15. Phosphorus Matches (Phosphorus P). Red/white striped book of 15, heads white, striker red (there are both white and red forms of phosphorus). The matches could light the dynamite (nitrogen) in the periodic table.
16. Sulfuric Acid (Sulfur S). One pint (16 oz.), pale yellow in a flask.
 17.Clorox (Chlorine Cl). Yellow-green bottle, the color of chlorine gas ("chloros" means yellow-green in Greek). Cleans 17 ways (Clorox is sodium hypochlorite). Note that Clorox has no letter "h" making it easy to remember the abbreviation Cl.
17.Clorox (Chlorine Cl). Yellow-green bottle, the color of chlorine gas ("chloros" means yellow-green in Greek). Cleans 17 ways (Clorox is sodium hypochlorite). Note that Clorox has no letter "h" making it easy to remember the abbreviation Cl.
18. Argon-filled Fluorescent Light (Argon Ar). 18-inch, sky-blue (air is 1% argon).
 19. Kettle, or Pot of gray Ashes (Potassium K). 19th-century way to make soap from potash (potassium is named for potash, potassium carbonate or hydroxide, which is named for the pot of ashes used to obtain it).
19. Kettle, or Pot of gray Ashes (Potassium K). 19th-century way to make soap from potash (potassium is named for potash, potassium carbonate or hydroxide, which is named for the pot of ashes used to obtain it). 20. Calcite Pearls (Calcium Ca). Ivory A string of 20 pearls. Calcite is a form of calcium carbonate.
20. Calcite Pearls (Calcium Ca). Ivory A string of 20 pearls. Calcite is a form of calcium carbonate.Atomic Numbers 1-20 41-60 61-80 81-105
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Atomic Number Memory Pegs: 21-40
 21. Scandal Page (Scandium Sc). One should be 21 years old to read the scandal page of the newspaper.
21. Scandal Page (Scandium Sc). One should be 21 years old to read the scandal page of the newspaper. 22. Titanic (Titanium Ti). The Titanic had 2200 passengers when it hit an iceberg and sank.
22. Titanic (Titanium Ti). The Titanic had 2200 passengers when it hit an iceberg and sank.
23. Chev Van (Vanadium V). 23-passenger van made with vanadium steel.

24. Chrome-plated Creamer (Chromium Cr). 24-oz. pitcher of cream
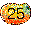
25. Autumn Mangos (Manganese Mn). 25 of them,

26. Ferris Wheel (Iron Fe). 26-seats and made of iron. Sounds like "ferrous," which refers to iron.

27. Cobblestone (Cobalt Co). It is a 3" cube, so it has a volume of 27 cubic inches.
 28. Nickel (Nickel Ni). In 1928, the year before the Great Depression, people had more money (a nickel) than in the next year ('29). The U.S. coin called a "nickel" used to be made of nickel.
28. Nickel (Nickel Ni). In 1928, the year before the Great Depression, people had more money (a nickel) than in the next year ('29). The U.S. coin called a "nickel" used to be made of nickel. 29. Copper Penny (Copper Cu). The Currency during the Great Depression which began in'29.
29. Copper Penny (Copper Cu). The Currency during the Great Depression which began in'29. 30. Brazen and zinc sink (Zinc Zn). Made of galvanized iron (iron coated with zinc), with $30 brass (brazen means brass) faucet. Brass is an alloy of copper and zinc. Sink sounds like zinc.
30. Brazen and zinc sink (Zinc Zn). Made of galvanized iron (iron coated with zinc), with $30 brass (brazen means brass) faucet. Brass is an alloy of copper and zinc. Sink sounds like zinc.
31. Galleon (Gallium Ga). The old Spanish galleons had three masts, each of which looked like a "1," to remind us of 31.
 32. German beer. (Germanium Ge) A quart stein (32 fl. oz.) of German beer. Germanium was named for Germany.
32. German beer. (Germanium Ge) A quart stein (32 fl. oz.) of German beer. Germanium was named for Germany. 33. A skull & crossbones (Arsenic As). Rat poison is arsenic trioxide, commonly called simply "arsenic." In other forms, such as is found in apples, arsenic is nutritious. Arsenic has three natural varieties: black, gray and yellow, as shown by the three colors in the icon.
33. A skull & crossbones (Arsenic As). Rat poison is arsenic trioxide, commonly called simply "arsenic." In other forms, such as is found in apples, arsenic is nutritious. Arsenic has three natural varieties: black, gray and yellow, as shown by the three colors in the icon. 34. Sensitive solar cell leaning (Selenium Se). Leaning at a 34° angle off vertical. Sounds like selenium. Solar cells often contain selenium.
34. Sensitive solar cell leaning (Selenium Se). Leaning at a 34° angle off vertical. Sounds like selenium. Solar cells often contain selenium.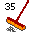 35. Broom (Bromine Br). 35 Bristles (seven rows of five). In the periodic table, note that all the elements in this column are cleaners.
35. Broom (Bromine Br). 35 Bristles (seven rows of five). In the periodic table, note that all the elements in this column are cleaners. 36. Krypton Yardstick. (Krypton Kr). One yard is 36 inches. Kryton gas is used to define the scientific unit of length, the meter, which is about a yard long.
36. Krypton Yardstick. (Krypton Kr). One yard is 36 inches. Kryton gas is used to define the scientific unit of length, the meter, which is about a yard long.
37. Superb ruby (Rubidium Rb). red ruby, 37-carat.

38. Israeli Strongbox (Strontium Sr). Bright red because the red in most fireworks is from strontium. It weighs 38 pounds.

39. A Tree (Yttrium Y). Y-shaped, with a "39" carved in it. Sounds like yttrium.

40. Ezra's Zircon Ring (Zirconium Zr). Only cost $40.
Atomic Numbers 1-20 41-60 61-80 81-105
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Atomic Number Memory Pegs: 41-60
 41. Unbearable Onion (Niobium Nb). "For one" person only. Sounds like Niobium and like "4,1". In Greek mythology, Niobe wept for the loss of her children, like an onion makes you weep.
41. Unbearable Onion (Niobium Nb). "For one" person only. Sounds like Niobium and like "4,1". In Greek mythology, Niobe wept for the loss of her children, like an onion makes you weep. 42. Molly's Denims (Molybdenum Mo). 42" waist. Sounds like Molybdenum.
42. Molly's Denims (Molybdenum Mo). 42" waist. Sounds like Molybdenum. 43. Dutch Technician (Technetium Tc). 43-years-old. Technetium was made synthetically; it is not stable enough to be found in nature.
43. Dutch Technician (Technetium Tc). 43-years-old. Technetium was made synthetically; it is not stable enough to be found in nature. 44. Ruth's hen (Ruthenium Ru). It's feet look like 44 and "Ruth's hen" sounds like Ruthenium. It is also by the wren and ostrich in the periodic table.
44. Ruth's hen (Ruthenium Ru). It's feet look like 44 and "Ruth's hen" sounds like Ruthenium. It is also by the wren and ostrich in the periodic table.
45. Road in Rhode Island. (Rhodium Rh). Speed Limit 45 m.p.h.

46. Top dog Palace (Palladium Pd). The sultan has 46 wives in its 46 towers, which look like 46.

47. Agnes's Silver Dime (Silver Ag). '47 was A Good year after WW II. (Silver is "argentum" in Latin).
 48. CD Caddy (Cadmium Cd) The caddy holds 4 dozen (48) CD's.
48. CD Caddy (Cadmium Cd) The caddy holds 4 dozen (48) CD's.  49. India Ink (Indium In). 49 cent bottle.
49. India Ink (Indium In). 49 cent bottle.  50. Tin Can (Tin Sn). Contains 50 Snails. Tin cans are made of steel sometimes covered with a thin layer of tin.
50. Tin Can (Tin Sn). Contains 50 Snails. Tin cans are made of steel sometimes covered with a thin layer of tin.
51. Ant money. (Antimony Sb). Taking small bills ($5 and $1) to its passbook account.
 52. Deck of 52 Cards (Tellurium Te). "Tell your" fortune. (Sounds like Tellurium)
52. Deck of 52 Cards (Tellurium Te). "Tell your" fortune. (Sounds like Tellurium) 53. Iodine (Iodine I). Bottle costs 53 cents.
53. Iodine (Iodine I). Bottle costs 53 cents. 54. Zenith television, watching "Xena" (Xenon Xe). On channel 54; a TV tube contains xenon gas.
54. Zenith television, watching "Xena" (Xenon Xe). On channel 54; a TV tube contains xenon gas. 55. Cesium Standard Clock (Cesium Cs). A physics clock. It reads 5:05; time to go home.
55. Cesium Standard Clock (Cesium Cs). A physics clock. It reads 5:05; time to go home.  56. Bad Berries (Barium Ba). Poisonous. About 5/6 of all white berries are poisonous (really). Black are usually edible.
56. Bad Berries (Barium Ba). Poisonous. About 5/6 of all white berries are poisonous (really). Black are usually edible.
57. Lantern (Lanthanum La). 57 candle-power.

58. Cereal bowl (Cerium Ce). Has 58 nutrients.
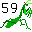
59. Praying mantis (Praseodymium Pr). Eats 59 bugs.

60. No Dim Lights (Neodymium Nd). Triangular sign has 60° angles. Bright lights can blind.
Atomic Numbers 1-20 21-40 61-80 81-105
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Atomic Number Memory Pegs: 61-80
 61. Prominent fire (Promethium Pm). Fire is needed after 6:10 p.m. Promethius brought fire to mankind. The flames look like 61.
61. Prominent fire (Promethium Pm). Fire is needed after 6:10 p.m. Promethius brought fire to mankind. The flames look like 61. 62. Small samurai sword (Samarium Sm). 62 generations of use.
62. Small samurai sword (Samarium Sm). 62 generations of use.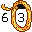
63. European rope (63 feet long). (Europium Eu).

64. Magdalene's Cattle (Gadolinium Gd). "Cattle" sounds like "gadol"inium. Checkerboard pattern (a checkerboard has 8x8 = 64 squares).

65. Bright blue turban (Terbium Tb). 65" long.

66. Dandy disposal (Dysprosium Dy). Disposes garbage in 6.6 seconds.

67. Home (Holmium Ho). This home has room for 6 or 7 children.
 68. Herb (Erbium Er). The stems and leaves form a hidden 68 in the picture.
68. Herb (Erbium Er). The stems and leaves form a hidden 68 in the picture.  69. Christmas Tulip (Thulium Tm). Red and green for Christmas. 69 kinds.
69. Christmas Tulip (Thulium Tm). Red and green for Christmas. 69 kinds. 70. Ladybug Litterbag. (Ytterbium Yb). A litterbag (small portable trash bag), which sounds like Ytterium, with a ladybug picture on it with 70 spots. Litterbags were popular in the '70s.
70. Ladybug Litterbag. (Ytterbium Yb). A litterbag (small portable trash bag), which sounds like Ytterium, with a ladybug picture on it with 70 spots. Litterbags were popular in the '70s.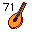
71. Lute (Lutetium Lu). 7 Strings on 1 Lute (71).
 72. Faithful Half Dollar. (Hafnium Hf). 72 is half a gross (of 144). Also, there are 72 ounces of silver in $100 of silver halves (or quarters or dimes). The ones dated 1964 or earlier are silver.
72. Faithful Half Dollar. (Hafnium Hf). 72 is half a gross (of 144). Also, there are 72 ounces of silver in $100 of silver halves (or quarters or dimes). The ones dated 1964 or earlier are silver.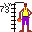 73. Tan Tall Man (Tantalum Ta). He is 7' 3" tall basketball player.
73. Tan Tall Man (Tantalum Ta). He is 7' 3" tall basketball player. 74. Westinghouse light bulb (Tungsten W). 74 Watt, with tungsten filament. Another name for tungsten is Wolfram, which explains the abbreviation.
74. Westinghouse light bulb (Tungsten W). 74 Watt, with tungsten filament. Another name for tungsten is Wolfram, which explains the abbreviation.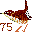 75. Wren (Rhenium Re). Visualize 75 of them on a telephone line.
75. Wren (Rhenium Re). Visualize 75 of them on a telephone line.  76. Ostrich (Osmium Os). 7'6" Tall. Also, its neck & head look like a 7 and its body like a 6.
76. Ostrich (Osmium Os). 7'6" Tall. Also, its neck & head look like a 7 and its body like a 6.
77. Iridescent Rainbow (Iridium Ir). 7 Colors in a rainbow (77). Iris was the Greek goddess of the rainbow.

78. Platinum Record (Platinum Pt). The old kind that rotated at a speed of 78 rpm. Recorded by "The Egyptians." A platinum recording sold more copies than a gold, and platinum is worth more than gold.

79. Gold Coin (Gold Au). A '79 A.U. (Almost Uncirculated.) The latin word for gold is aurum.

80. High-grade Thermometer (Mercury Hg). Showing 80 degrees.
Atomic Numbers 1-20 21-40 41-60 81-105
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Atomic Number Memory Pegs: 81-105
 81. Tallow Candle (Thallium Tl). 81. Looks like a "1" and sounds like "tallow." Used in castles.
81. Tallow Candle (Thallium Tl). 81. Looks like a "1" and sounds like "tallow." Used in castles.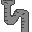 82. Lead Plumbing (Lead Pb). Under the cupboard. 82% lead. The latin word for lead is plumbum.
82. Lead Plumbing (Lead Pb). Under the cupboard. 82% lead. The latin word for lead is plumbum.
83. Pepto Bismol (Bismuth Bi). I "ate 3" teaspoons of Pepto Bismol (sounds like "83"). Pepto Bismol contains Bismuth Subsalicylate.
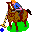
84. Polo Stick (Polonium Po). 7 dozen (84) polo sticks.
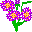
85. A tall Aster [85 petals] (Astatine At). The flowers, stem and leaf form an "85".

86. Radar warning. (Radon Rn). Protection for 86 miles.
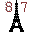
87. France's Eiffel Tower (Francium Fr). Built for the World's Fair and commemorated the centenniel of the French Revolution which technically began in 1787.
 88. Radio (Radium Ra). Piano music (88 keys) on FM 88.
88. Radio (Radium Ra). Piano music (88 keys) on FM 88.  89. Actor (Actinium Ac). He won 89 oscars.
89. Actor (Actinium Ac). He won 89 oscars.  90. Thorn (Thorium Th). Thorns stick out at 90° angle.
90. Thorn (Thorium Th). Thorns stick out at 90° angle. 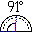
91. Paper protractor (Protactinium Pa). Measuring 91°, one degree more than a right angle. Use it to measure the angle of the thorns next to it.
 92. Uranium A-Bomb. (Uranium U). 92 Kilotons. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94.
92. Uranium A-Bomb. (Uranium U). 92 Kilotons. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94. 93. Neptunian pitchfork (Neptunium Np). Neptune's trident is 9' long with 3 prongs. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94.
93. Neptunian pitchfork (Neptunium Np). Neptune's trident is 9' long with 3 prongs. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94. 94. Pluto the Pup (Plutonium Pu). Mickey Mouse's dog. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94, and remember that Pluto's ears look like a 94.
94. Pluto the Pup (Plutonium Pu). Mickey Mouse's dog. Memorize Uranus, Neptune and Pluto (the planets in a row) for 92, 93, 94, and remember that Pluto's ears look like a 94. 95. American flag (Americium Am). Cost $95 .
95. American flag (Americium Am). Cost $95 . 96. Acme's curious curtains (Curium Cm). For a standard 8-foot tall room (96").
96. Acme's curious curtains (Curium Cm). For a standard 8-foot tall room (96").
97. Rib knit Beret (Berkelium Bk). '97 style. Named for Berkeley, California, where berets have been seen.

98. Cauli-flower (Californium Cf). Remember Berkeley, California, are consecutive and that there is a 98 hidden in the cauliflower. California produces a lot of cauliflower.

99. Einstein's esoteric earrings. (Einsteinium Es). The hidden meaning is that his theory predicts you can only go 99% of the speed of light.

100. Fur mink (Fermium Fm). "Fur" sounds like fermium. It cost 100 thousand dollars and is 100% real fur from mink.

101. Mental M.D. (Mendelevium Md). They work on brains. Mental MD's all took Psychology 101.

102. No Bell (Nobelium No). The red bar divides the picture into two parts, to remember 102.

103. Laurel Wreath. (Lawrencium Lr). It has 103 leaves, and this one was also made with bulrushes.

104. Ruth's perfect Ford. (Rutherfordium Rf). It has 4 wheels and Ford sounds like "four" to remember 104. The Model-T was so "perfect" that is was popular for many years.

105. Hand. (Hahnium Ha). Has 5 fingers, for 5 more than 100
.
Atomic Numbers 1-20 21-40 41-60 61-80
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Using Atomic Number Memory Pegs
Many memory aid systems use memory "pegs," which are pictures used to remind you of numbers.- First, memorize the first twenty pictures, especially the association with the numbers.
- Then, to memorize twenty objects in order, simply think of a picture using each item with one of these 20 previously memorized pictures, and it is easy to remember the objects in order.
For example, using the fire hydrant picture for number one, you could think of the first object as being balanced on top of a hydrant, or being soaked by a hydrant, or perhaps with a hydrant on top of the object.
When I took a memory pegs course, I was struck by how senseless the twenty items were, that we were told to memorize, so I tried to think of a list of numbers that are identified with something in nature. My answer is to use the Atomic Numbers! Every element in nature has a number associated with it called the atomic number. I have thought of a set of pictures for 105 objects, which has these features:
- Each picture reminds you of the element's name.
- Each picture reminds you of the number.
- Each name or description reminds you of the element's chemical symbol.
The first twenty, which are the most important elements and also most common numbers, also have these two features:
- Each picture is an example of the use of that element.
- Each picture is represented by a different color.
That is, the most common organic elements are oxygen, carbon, nitrogen and hydrogen and the have the common colors: white, black, red and blue.
The abbreviations are in bold, the colors are in italics, and similar sounds are underlined.
When you memorize them, it is important to
- associate the number with the picture, and then as a secondary step,
- associate the picture with the element.
Atomic Numbers 1-20 21-40 41-60 61-80 81-105
SOURCE: John P Pratt (Mormon)
Subscribe to:
Posts (Atom)